Blood Matters
Providing a safe and adequate supply of blood is vital to the lifesaving work done in healthcare systems.
Analysis, planning, validation, training, and ongoing quality assessments are all part of the quality systems that blood establishments routinely employ to ensure controlled implementation of changes and new technologies.15 PI has been instituted under such rigor in over 30 countries. Care is taken to address risks as they are identified, and solutions are weighed against their cost and benefit. But, what if time is not on one’s side?
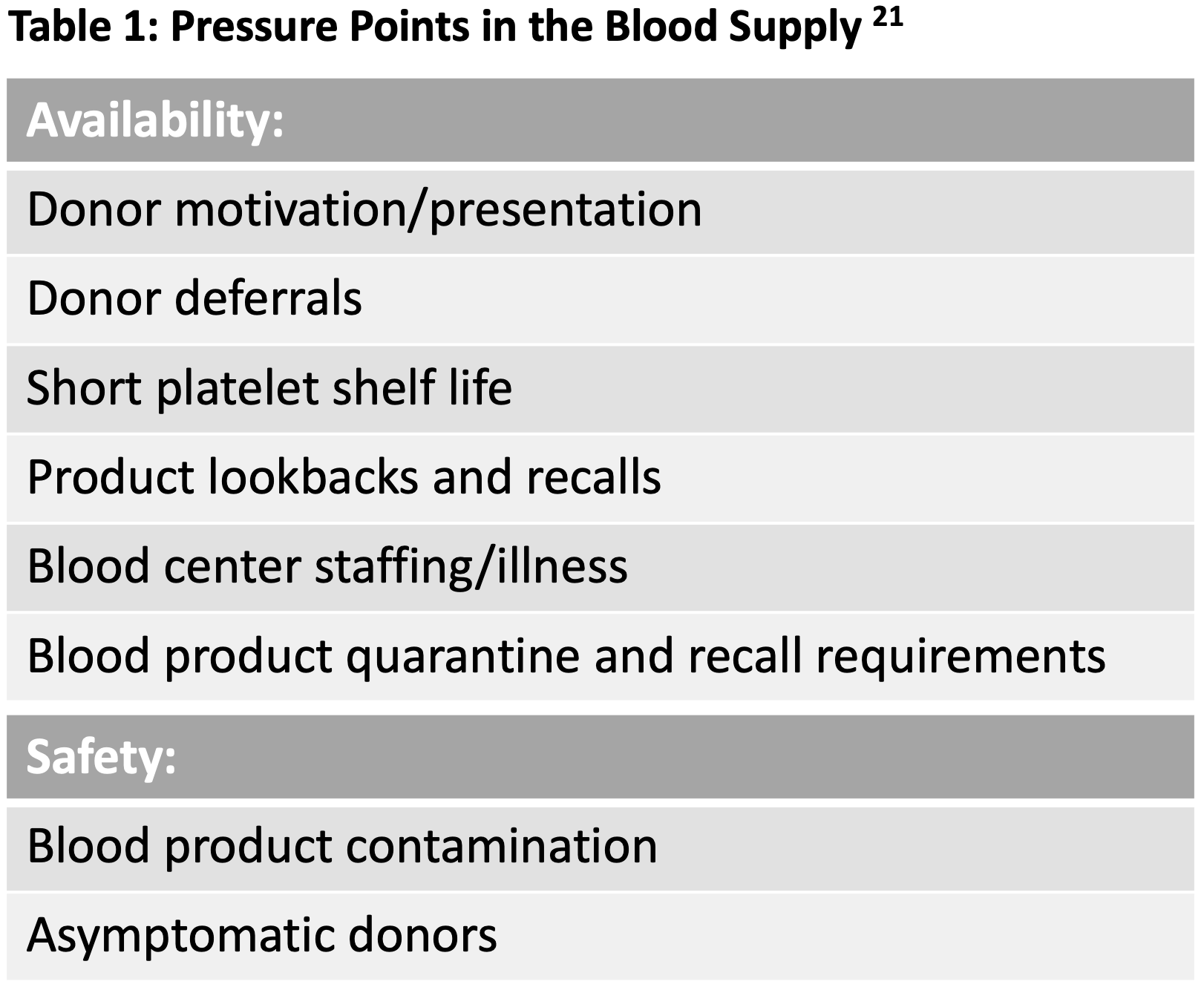
In the field of blood transfusion, specialists continually navigate a supply chain riddled with pressure points (Table 1)21. Processes and technologies are deployed in concert to address these pressures, but not every solution can sufficiently tackle the enormity of a novel threat. To avoid contaminated units, one might defer donors potentially exposed to infectious agents, but this can severely limit donations and impact availability. During a pandemic, an everyday challenge can become crippling.
1 Blumenthal D, Fowler E. It’s not too early to prepare for the next pandemic. HBR. Accessed May 4, 2020. https://hbr.org/2020/04/itsnot-too-early-to-prepare-for-the-next-pandemic
2 UN Department of Economic and Social Affairs. Everyone Included: Social Impact of COVID-19. Accessed May 8, 2020. https://www.un.org/development/desa/dspd/everyone-included-covid-19.html
3 Gallian P, et al. PLoS neglected tropical diseases 2017;11:e0005254-e
4 AABB 2015. Intercept-treated platelets from CHIKV positive donations were transfused to 10 recipients with no clinical evidence for transmission. Abstract # S26-020B. Transfusion 2015 Suppl. 3, 17A
5 Rezza, 2018. J Travel Med: doi: 10.1093/jtm/tay004
6 Rasongles P, et al. Transfusion 2009;49:1083-91
7 Cerus Corporation. INTERCEPT Platelets: Technical Data Sheet. Accessed May 5, 2020. https://www.interceptbloodsystem.com/sites/default/files/resources/prd-tds_00121_v11.0.pdf
8 ECDC. Dengue outbreak in Réunion, France, and associated risk of autochthonous outbreak in the EU/EEA. 18 June 2019. Accessed May 5, 2020. https://www.ecdc.europa.eu/sites/default/files/documents/RRA-dengue-Reunion-18-June-2019.pdf
9 WHO. Interim Guidance: Maintaining a safe and adequate blood supply during Zika virus outbreaks. February 2016. Accessed May 5, 2020. https://www.who.int/csr/resources/publications/zika/Safe-blood_supply18Feb2016.pdf
10 ECDC. Local transmission of dengue fever in France and Spain – 2018. 22 October 2018. Accessed May 5, 2020. https://www.ecdc.europa.eu/sites/portal/files/documents/08-10-2018-RRA-Dengue-France.pdf
11 PEI, Stufenplan Stufe 2. Accessed March 18, 2020 at: https://www.pei.de/SharedDocs/Downloads/DE/arzneimittelsicherheit/haemovigilanz/anhoerungen/2019-12-23-anhoerung-wnv-stufe2.pdf?__blob=publicationFile&v=2
12 Cazenave JP, Waller C, Kientz D, Mendel I, Lin L, Jacquet M, et al. An active hemovigilance program characterizing the safety profile of 7,483 transfusions with plasma components prepared with amotosalen and UVA photochemical treatment. Transfusion. 2010;50:1210-9.
13 Dean CL, Hooper JW, Dye JM, Zak SE, Koepsell SA, Corash L, et al. Characterization of Ebola convalescent plasma donor immune response and psoralen treated plasma in the United States. Transfusion. 2020.
14 Geisen C, Kann G, Strecher T, et al. Pathogen-reduced Ebola virus convalescent plasma: First steps towards standardization of manufacturing and quality control including assessment of Ebola-specific neutralizing antibodies. Vox Sanguinis. 2016;110(4).
15 General European OMCL Network (GEON): Quality Management Document. PA/PH/OMCL (16) 122. Change Control Accessed May 5, 2020. https://www.edqm.eu/sites/default/files/recommandation-omcl_16_122_r_change_control_web_publication.pdf
16 WHO. Disease Outbreak News. Accessed May 5, 2020. https://www.who.int/csr/don/en/
17 Pierelli et al. TRANSFUSION 2018; 58, pp 3027. Emergency response of four transfusion centers during the last Chikungunya outbreak in Italy.
18 FDA Recommendations for Investigational COVID-19 Convalescent Plasma. Updated April 13, 2020. https://www.fda.gov/vaccinesblood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19- convalescent-plasma#Pathways%20
19 Global Preparedness Monitoring Board. A World at Risk: Annual report on global preparedness for health emergencies. September 2019. Accessed May 5, 2020. https://apps.who.int/gpmb/assets/annual_report/GPMB_annualreport_2019.pdf
20 Lewis P. Legal Provision for Crisis Preparedness: Foresight not Hindsight. Chatham House. 21 April 2020. Accessed May 5, 2020. https://www.chathamhouse.org/expert/comment/legal-provision-crisis-preparedness-foresight-not-hindsight#
21 Cerus internal resources
